In My Classroom: POOP LAB! (AKA Fecal Floats/intestinal parasite lab)

A lab favorite in my classroom is the Poop Lab. I teach a Veterinary Medicine course for high school juniors and seniors (life science credit). Parasitology is one of the class foci (is it because I LOVE the gross factor? Probably). If you cover parasites, zoology or even zoonotic diseases, this lab is a must do! YOU DO NOT NEED A CENTRIFUGE to do this lab. Alternative instructions are written on the student lab.
Goal of the lab:
Perform a fecal float analysis with sample from a family pet. Determine if that pet has possible intestinal parasites.
Rationale: Determining if the students’ own pet has a parasite is extremely engaging (or the neighbor’s pet). Plus, it’s a darn good microscopy
exercise on identifying what is significant or not (hair or air bubble).
Homework the night before: I send students home with a disposable fecal collection chamber (1). If you don’t have those, a Ziploc or other small disposable container will suffice (the thicker the better stink barrier it will be). I also give them the option of grabbing a set of exam gloves in case they are horrified by the thought of poo grazing their hand. Students are instructed to collect 2-5 cc of FRESH ANIMAL poo from their pet at home (or ask a neighbor for use of their yard/litter box/etc.). No human poo accepted. ANY pet will work. Iguana, dog, chicken, horse, cat, goat, hamster, chinchilla, etc.

I’ve usually emailed home to give the parents a heads up that bringing an ANIMAL poop sample to school is legit (no worries about stuffing it someone else’s locker as a prank). FRESH IS BEST so collection morning of the lab preferred, but most animals don’t poop on command. If they have an evening pooping animal, it’s OK to collect the night before, but should be refrigerated overnight (um, this is again where a parental heads up comes in handy – probably won’t want container of poop in the family fridge). If poop is banned from the kitchen, a lunch box with freezy pack. If they use ice to keep it cool, make sure the water doesn’t leak into the poo container. That is a lunch box sludge that is best avoided.
Lab Day: If this is not your students’ first time using a microscope, this lab can be completed in a 45 minute session, including cleanup. This is possible because I prefer to maximize the use of disposable items. After the first student spills a test tube of sugared poo slurry and you see how difficult it is to clean up the sticky mess, you’ll thank me for that little tidbit of wisdom. Try asking your local Sonic, etc. for a donation of a box of smoothie straws (AKA disposable scoopulas) to mentally scar students’ future enjoyment of smoothies following this lab.
Here’s the one-page handout of instructions that I give each student:

I built tips into the instructions, so give it a good read before trying with students. You will need a comparison chart [(2) or extension activity described below BEFORE this lab] so that if students find something that looks like a parasitic egg (4), they can try to identify which parasite might be infesting Fluffy’s intestines.
You will need to order or mix up a sugar solution ahead of time (3). Wards has a “Fecal Slide Analysis Activity” which boils down to this same lab. I wouldn’t spend the money on it (although I did the first year), unless you like the handy dandy teacher manual (which does have some interesting fun facts – but that’s all I like about it).
How it works: The parasite eggs (4) are less dense than the solution. After centrifuging (or allowing to sit for a length of time), the eggs float to the top of the tube. They bind to the coverslip, allowing us to see the eggs on the scope. The parasitic infection can be determined based on the shape/appearance of the eggs on the slide.
IF there is anything suspect, use an identification chart (2) or a scientific based website to try to match the microscope image with known parasite pictures. See additional activity ideas below. Check out this website:
http://www.pet-informed-veterinary-advice-online.com/fecal-float.html I like that towards the bottom, they also identify other items which might be seen on the slides and are no cause for alarm (normal bacterial flora, hairs, air bubbles).
My students have seen green blobs that look like stacked bricks. It’s a hoot for them to try to guess what network type of parasite that might be, only to finally draw the connection between plant cells from Bio I and, “oh yeah, my dog ate grass.”

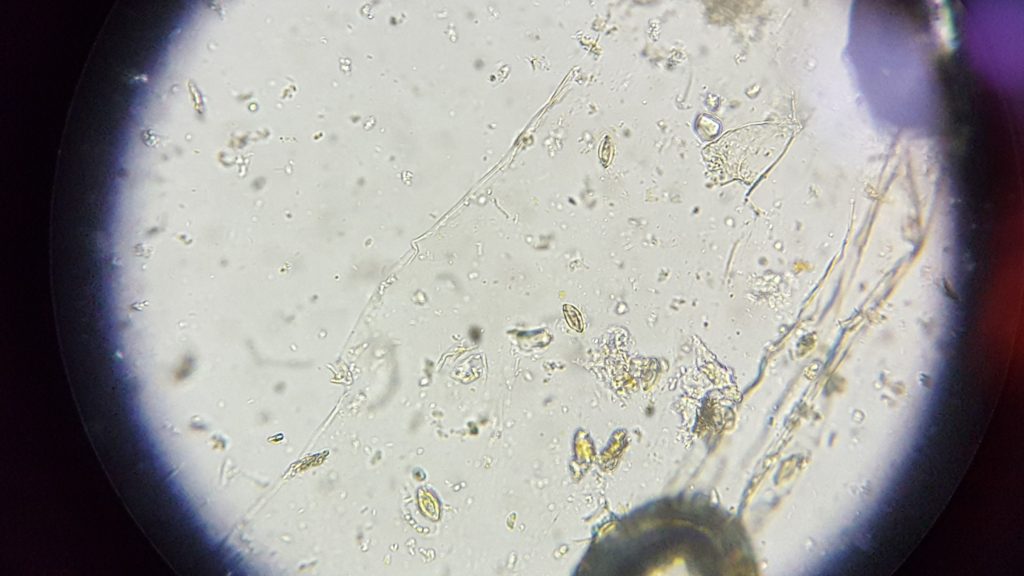
Note about livestock: It is actually considered normal for a livestock animal to have some parasitic load. This keeps the immune system fighting the parasites instead of treating again and again to the point they become resistant. Instead of a fecal float (like this lab) for diagnosis, a count is performed after isolating possible eggs. A cell count slide is used (sounds like a hemocytometer to me). If 1 cc of fecal matter yields over a certain threshold for that parasite in that animal, then treatment is administered. Don’t ask me the threshold – if you come across any more info about this, please add to the comment section below. Fall 2018, my students found Strongyles (a horse nemotode) in the poo I brought in from one of my horses. I showed the picture to my veterinarian. Although we didn’t quantify, she felt it was probably a low parastitic load and would naturally be kept at bay. I rotate through a different dewormer for my horses every spring and fall. Horse strongyles can’t be passed to my happily horse poop eating hound dogs, so they continue to be clear every time my class looks at their poop.
What to do if evidence of parasite IS found in a pet:
Instruct the student not to panic. Have the student take a photo of the microscope image. It is recommended that he/she share the information with the parent to possibility be passed along to the family veterinarian. The vet while likely ask for a fecal sample or to bring the pet to the clinic for a fresh sample to be taken (via tiny spoon inserted into the anus – very fresh). Some veterinarians might do a version of an ELISA to confirm various parasites. After doing this 10 semesters in our suburban school setting, I have only had one pet fecal sample come out positive (Coccidia). That was a sample collected the morning of class from an abandoned poo pile in the student’s yard. Honestly, the HUNT for parasitic evidence is fun – you really don’t want a positive result. That means the pet *likely* has a parasite.

Extensions / additional activities pre or post lab:
- BEFORE the lab – Have students research a parasite (5), add summary (signs of that disease, species affected, is it zoonotic, is a vector involved/how, long term health effects if untreated, etc.) PLUS microscope image to a Google sheet. Use that sheet as a reference if students see something suspect on lab day. Merck Veterinary Manual Online (free) gives good and easy to understand descriptions of the parasitic diseases.
https://www.merckvetmanual.com/ . The CDC has awesome info about zoonotic parasites. I especially love the life cycles.
https://search.cdc.gov/search/?query=intestinal+parasites&sitelimit=&utf8=%E2%9C%93&affiliate=cdc-main - AFTER the lab – Now that you have them hook-wormed (see what I did there?), do an activity on life cycles. I have created a Direct vs. Indirect Life Cycle activity using manipulatives which I’ll share at a later date.
- BEFORE OR AFTER – Dissect Ascaris worms.
https://www.carolina.com/preserved-other-animals/formalin-preserved-ascaris-/FAM_224405.pr?question=parasitic
References to above

(1) – Ordering info for collection chambers – the “official” fecal collection chambers aren’t needed. A disposable conical lab tube (with lid) would be fine. However, if you want the “real thing,”
- JorVet labs ($25 for 50 chambers).
https://www.jorvet.com/product/fecal-ova-float-kits-wo-dispensing-box/ - Amazon has either style. The chambers on the right of my photo were $125 for 500 chambers.
(2) – Identification chart: You might be able to obtain some charts through your local veterinarian OR make your students create a chart as a pre-lab (described in extension activities). I have found that Pinterest has several charts, found through a quick Google search (make sure you’re looking at companion animals, not just goat parasites):
https://www.google.com/search?q=veterinary+parasite+identification+chart&safe=active&sa=X&rlz=1C1GCEA_enUS762US762&biw=1242&bih=568&tbm=isch&source=iu&ictx=1&fir=0UCOnjmsSngtCM%253A%252CiMdeL3n_rdcDwM%252C_&vet=1&usg=AI4_-kT8I1O40h9HcKINDu9L7M-xel3U9Q&ved=2ahUKEwiugub5osbhAhXLs54KHQJrCwsQ9QEwAHoECAkQBA&scrlybrkr=11de4934#imgrc=3ShBbbz1RdtkaM:&vet=1
(3) – Ordering info for Sheather’s Fecal Float (sugar) Solution – you can mix this up yourself with sucrose and dWater. The exact specific gravity should be 1.27. The solution is described in Dr. Dryden’s article below (Magnesium Sulfate solution can be used instead of sugar, also described in the article). Alternatively, you can order the solution pre-made.
- Dryden MW, Payne PA, Ridley R, et al. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther2005;6(1):15-28.
- JorVet labs ($25 for a gallon which should last all year).
https://www.jorvet.com/product/sheathers-sugar-flotation-gallon-bottle/
4) – “Eggs” are used generally in this article. Depending on the parasite, students may actually be looking at/for the actual protozoa (Giardia trophozoite), the egg (tapeworm or hookworm oocyte) or cyst (Giardia cyst – tenacious temperature tolerant oocyte manifestation).
(5) – Possible companion animal parasites include: Alaria (intestional flke), Spirometra (taepworm), Paragonimus kellicotti (lung fluke), Platynosomum fastosum (liver fluke), Dipylidium canium (flea tapeworm), Taenia species (tapeworm), Capillaria aerophila (lungworm), Trichuris vulpis (whipworm), Uncinaria stenocephala (hookworm), Ancylostomas species (hookworm), Physaloptera species (stomach worm), Toxascaris lonina (round worm), Toxocara cati (roundworm), Toxocara canis (round worm), Baylisascaris species (racoon roundworm), Strongyloides larvae (threadworm), Giardia trohphozoite, Giardia cyst, Isospara species (Coccidia), or Toxoplasma gondii.
Additional resources:
- Companion Animal Parasite Council (CAPC): http://www.petsandparasites.org/resources/pets-parasites-and-people Clicking on the various parasite names gives you more info about each AND some links have YouTube videos of the parasites tooling around. Disguestingling awesome.
- Kansas State University, School of Veterinary Medicine: https://www.vet.k-state.edu/vhc/services/phc/common-parasites.html
- Safepath Laboratories, Five Most Common Intestinal Parasites in Dogs & Cats: https://safepath.com/five-most-common-intestinal-parasites-in-dogs-and-cats/
